
India needs to focus on reducing dependency on single sources by qualifying other geographies
There are several challenges in the traceability of the pharma supply chain. They mainly consist of controllable factors such as managing shipment time, lead time, temperature excursions, and maintaining quality checks, and uncontrollable factors such as the Red Sea Crisis increased lead time and planning.
Companies and the industry need to work towards enhancing end-to-end traceability by improving forecast accuracy and ensuring adherence to compliance. Efforts must be taken to reduce lag/lead time, avoid temperature excursion, and ensure that all related documentation is completed and filled correctly. India pharma Inc. needs to focus on reducing dependency on imports and dependency on single sources by qualifying other geographies. In order to ensure quality control, supplier qualifications must include quality testing (Nitrosamine + other tests from national/government labs of the country).
Available solutions
◆ Track and trace mechanisms to be installed and monitored with validation in place
◆ Datalogger real-time online tracking/monitoring
◆ Temperature challenge: Pharma companies focus mainly on cold chain and ensure that the products are transported across the length and breadth of the country using cold chain transportation. In India, we are seeing a boom of reefer vehicles which are being used across industries.
◆ Depute 'speciality couriers' rather than the regular freight forwarders. Speciality couriers have a very tight TAT and monitoring mechanism from end to end. A few companies to name, PDP, Ontime Globe, Alisped etc
◆ Companies have moved from traditional USB data loggers to smart IOT-based loggers which give real-time monitoring of temperature during the transit of the products and storage in warehouses.
This story is from the December 2024 edition of Express Pharma.
Start your 7-day Magzter GOLD free trial to access thousands of curated premium stories, and 9,000+ magazines and newspapers.
Already a subscriber ? Sign In
This story is from the December 2024 edition of Express Pharma.
Start your 7-day Magzter GOLD free trial to access thousands of curated premium stories, and 9,000+ magazines and newspapers.
Already a subscriber? Sign In

CILICANT is changing the way we think about active packaging
Team CILICANT shared insights into their journey of innovation, customer-centric solutions, and their ambitious plans for global expansion. In this engaging dialogue, the team also shed light on their unique value proposition, recent achievements, and strategies for adapting to a rapidly evolving pharma landscape, in an exclusive conversation with Express Pharma
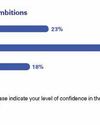
KPMG 2024 Life Sciences CEO Outlook
This recent KPMG report explores the perspectives and priorities of life sciences CEOs amid economic and geopolitical uncertainty, focusing on growth strategies, digital transformation, ESG goals, and workforce challenges. Excerpts from the report

Nutraceuticals 2025: Mapping growth, innovation, and consumer-centric trends
Industry experts highlight the evolving dynamics, regulatory needs, and personalised solutions driving the nutra market ahead of 2025

We aim to strengthen our presence in Asian and MENA regions, with particular emphasis on India
Matthias Poslovski, Chief Sales Officer, Optima Pharma, speaks on the company's evolution and shares insights on emerging trends like personalised medicine, digitalisation, and Al-driven technologies, while outlining his company's strategies for market expansion, particularly in Asia and MENA, with a focus on the Indian pharma sector, in an exclusive interaction with Lakshmipriya Nair

Sustaining excellence: The evolving role of quality in pharma
In the ever-evolving landscape of the pharma industry, the pursuit of quality remains a cornerstone of success. As stringent regulatory frameworks, rising patient expectations, and the increasing pace of innovation, transforms the pharma industry, the definition of quality is also evolving. From ensuring compliance with global standards to embracing cutting-edge technologies like Al-driven quality control, the industry is navigating a paradigm shift in how it approaches product integrity, safety, and efficacy. In this New Year issue, pharma experts explore how crucial it is to foster a culture of quality across organisations and deliver better healthcare outcomes

Revolutionising pharma the adaptive way
Adaptive manufacturing offers better performance, efficient management of diverse products, a reduced machine footprint, faster batch changes, and enhanced operational sustainability

Trends 2025: Redefining the pharma supply chain
Saurabh Gupta, Global Head of Supply Chain, Lupin highlights trends such as Al integration, digitalisation and sustainability that are shaping a future-ready supply chain

Growth mantras for 2025: Innovation, Partnerships, Sustainability
Innovation, synergy, and sustainability are shaping the trajectory of India's pharma sector, unlocking new frontiers of growth, inform industry stakeholders

Will Epygen's biosimilars bets pay off?
Debayan Ghosh, founder, Epygen Group, has ambitious plans to snag a slice of the booming $70 billion global biosimilar pie. In a conversation with Viveka Roychowdhury, he analyses the journey so far, spanning pandemic preparedness projects like protein-based vaccines, building up pipeline revenue streams in diabetes, oncology, immunology and cardiovascular biosimilars, and his strategies to scale up and expand to other therapeutics like anti-obesity drugs

Innovation, partnerships and best practices: Priorities for biopharma in 2025
Industry experts highlight the progress made by the Indian biopharma sector, and suggest pathways to promote growth